【中文翻译03】ISPE 良好实践指南:制药行业的知识管理
2023-12-26 73
【英文】ISPE Good Practice Guide: Knowledge Management in Pharmaceutical Industry;【中文】ISPE 良好实践指南:制药行业的知识管理;发布时间:2021年5月;指南页数:160页;指南章节数:23章
中文翻译03:1. 引言(1.7)
1 Introduction
1 引言
1.7 Key Concepts and Terms
1.7 关键概念和术语
1.7.1 Knowledge Management
1.7.1 知识管理
ICH Q10 [4] defines Knowledge Management as:
“Knowledge management is a systematic approach to acquiring, analysing, storing and disseminating information related to products, manufacturing processes and components. Sources of knowledge include, but are not limited to prior knowledge (public domain or internally documented); pharmaceutical development studies; technology transfer activities; process validation studies over the product lifecycle; manufacturing experience; innovation; continual improvement; and change management [italics in original] activities.”
The focus of this ICH Q10 [4] definition is on documented knowledge. A more holistic definition comes from APQC 1 [9]:
“The application of a structured process to help information and knowledge flow to the right people at the right time so they can act more efficiently and effectively to find, understand, share, and use knowledge to create value.”
This definition is commonly used by KM practitioners because it embraces the two main aspects of KM: the needs of the knowledge user and the needs of managing knowledge within an organization.
A useful thought model when considering the role of KM in organizations is that of knowledge and knowledge recognition. Figure 1.1, depicts whether an organization possesses the knowledge (i.e., does the knowledge exist) versus knowledge recognition (i.e., are we aware it exists). While this may seem relatively simple at an individual level, it is much more complex at an organizational level across many people, functional areas, products, processes, etc.
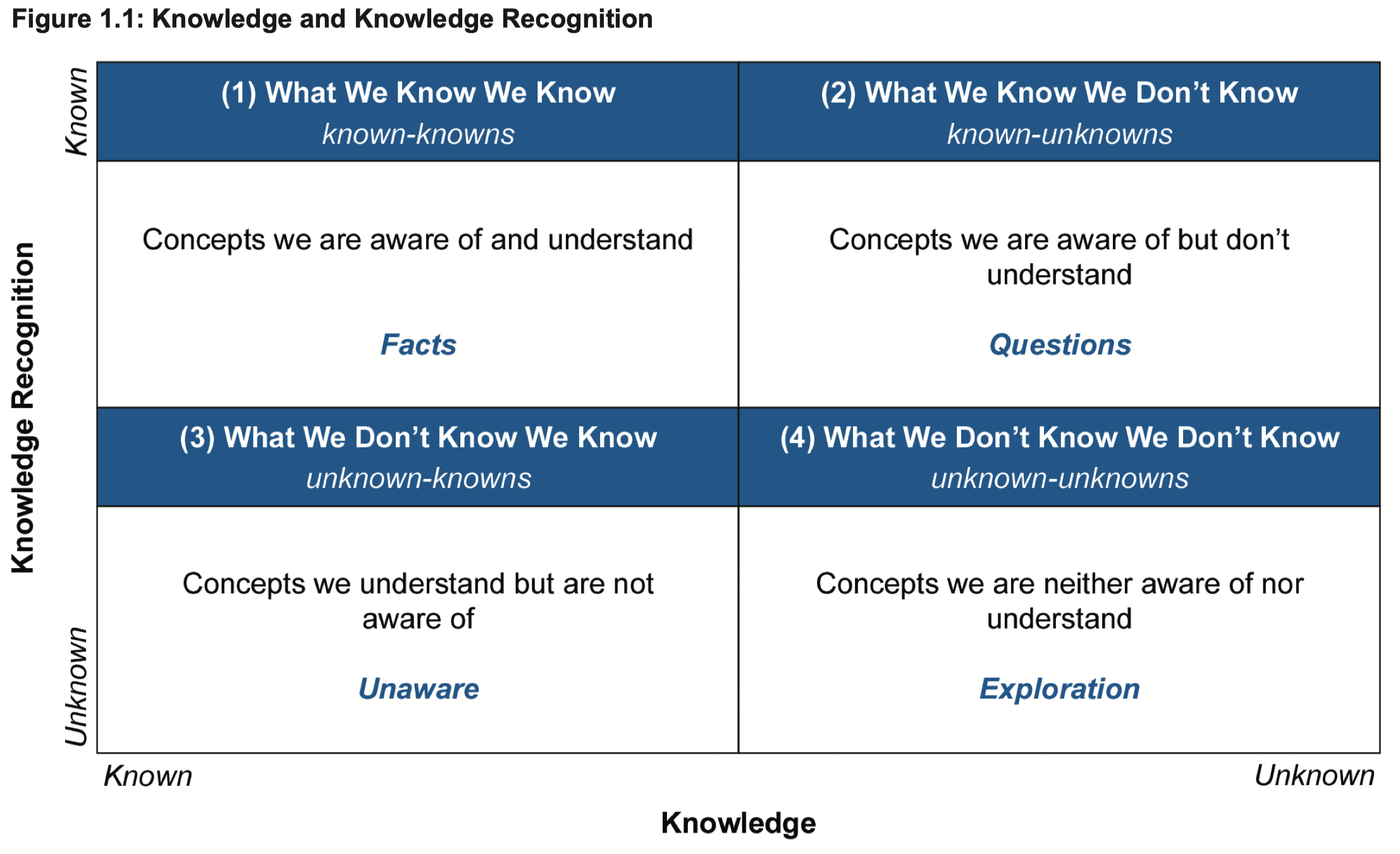
- Arguably the most central role of KM begins with ensuring effective management of Box 1 in Figure 1.1, described as Facts (inclusive of tacit knowledge). We know what we know and can find this knowledge when and where needed. An example of this is applying prior knowledge at the start of development of a new product.
- 【我们知道自己知道】可以说,知识管理最核心的作用开始于我们能确保对图1.1中第一象限的知识进行有效管理,即事实(包括隐性知识)。事实知识,即我们知道我们所知道的,我们可以在需要的时间和地点找到这些知识。这方面的一个例子就是我们在新产品开发之初所应用的已有先验知识。
- Box 2 in Figure 1.1, described as Questions, represents instances where we are aware we do not have sufficient knowledge. Experimentation is directed to answer such questions and grow the body of knowledge. Risk management also plays a role in how risks associated with unknowns are managed. As these questions are answered, this knowledge should be managed effectively henceforth, including what did not work or other unanticipated learnings (in Box 1).
- 【我们知道自己不知道】图 1.1 中第二象限的知识,我们通常描述为“问题”,表示我们能够意识到的自己并不足够掌握的知识。我们往往通过实验来回答这些问题并积累沉淀知识体系。风险管理也在该象限中,如何管控与未知因素相关的风险方面,发挥着作用。而随着这些问题被解答,这部分知识今后应该得到有效的管理,包括解答过程中获得的并不奏效的知识或其他预期外的学习成果(都应进入象限1)。
- Box 3 in Figure 1.1, described as Unaware, represents untapped potential and knowledge loss within an organization, such as when expertise exists in an organization to solve a problem, but the connection is never made, or knowledge is lost due to the departure of key personnel or during transfers between different teams in a product’s lifecycle. Tacit KM techniques such as lessons learned, communities of practice, tacit knowledge retention and transfer processes can play a key role in creating dialog that can lead to new insights. This knowledge should be codified and shared as appropriate (and therefore in Box 1). An effective systematic KM program within an organization’s PQS is a key administrative tool to help minimize such knowledge loss and maximize knowledge awareness.
- 【我们不知道自己知道】图 1.1 中第三象限的知识,我们通查描述为“无意识”,表示组织内未开发的潜力和知识流失,比如,当组织中存在能够解决问题的专业知识,但这些知识从未与组织建立联系,或者由于关键人员的离开,再或者产品全生命周期中不同团队之间的知识转移问题,而造成了知识流失。在面对一些隐性知识时,比如经验教训、集体实践等,我们需要有一些管理这部分知识的技巧,而所谓的留存和转移隐性知识的整个过程,可以为创造组织内集体讨论发挥关键作用,从而促使组织产生出新的见解。这些隐性知识应根据实际情况被记录、编纂、分享(因此应进入第一象限)。在组织的PQS中,一个有效的系统性知识管理计划是一个关键的管理工具,可以帮助组织最大限度地减少类似这种知识流失,并最大限度地提高组织的知识管理意识。
- Box 4 in Figure 1.1, described as Exploration, represents the opportunity for ongoing learning and knowledge acquisition whether intentional (experimentation or other directed recognition efforts) or unintentional (investigations resulting from process deviations). This new knowledge may lead to new Questions and new Facts and should be managed appropriately.
- 【我们不知道自己不知道】图 1.1 中第三象限的知识被描述为“探索”,代表了一个组织持续学习并获取知识的机会,无论这些机会是有意为之(比如通过实验或其他有针对性的识别工作)或是无意为之(比如由过程偏差引发的调查)。这些新知识可能会带来新的事实(第一象限)或新的问题(第二象限),都应该进行合适的管理。
1.7.2 Types of Knowledge
1.7.2 知识的类型
Explicit Knowledge: A declarative type of knowledge that can be readily articulated (in words or images), coded, stored, and accessed.
Tacit Knowledge: A context-specific type of knowledge, acquired through personal experience or internalization that resides within people’s minds rather than any physical media or information system. Often referred to as know-how.
An important theme throughout this Guide is the emphasis on tacit knowledge. Given the regulated and document-centric nature of the pharmaceutical industry, the importance and impact of tacit knowledge is arguably underappreciated. This Guide aims to highlight this, and where appropriate, provide KM methods and tools to better recognize, capture, transfer, and apply tacit knowledge.
Explicit and tacit knowledge are explored further in Section 3.2.
1.7.3 Additional Terms
1.7.3 其他术语
Principles and additional key concepts for KM are discussed in Chapter 3.
Knowledge Management Methods and Tools: Structured and standardized solutions to facilitate effective knowledge management. Also known as KM approaches, capabilities, or practices.
Knowledge Workers: Workers who think for a living and use expertise, education, and experience to solve work problems through the creation, distribution, or application of knowledge. The pharmaceutical industry is considered a knowledge industry and as such, its workforce is comprised of knowledge workers.
Lessons Learned: The concept of Lessons Learned is often referred to as After Action Review. This Guide treats them as synonymous and will refer to the activity as Lessons Learned.