【中文翻译04】ISPE 良好实践指南:制药行业的知识管理
2023-12-26 206
【英文】ISPE Good Practice Guide: Knowledge Management in Pharmaceutical Industry;【中文】ISPE 良好实践指南:制药行业的知识管理;发布时间:2021年5月;指南页数:160页;指南章节数:23章
中文翻译04:2. 知识管理:药品质量体系与运营效率的关键推动力(2.1)
2 Knowledge Management: A Critical Enabler to the Pharmaceutical Quality System and Operational Effectiveness
2 知识管理:药品质量体系与运营效率的关键推动力
Effective KM is an expectation established in a number of regulatory guidance documents, most notably the ICH series of Quality Guidelines [1]. This chapter explores the importance of KM positioned as an enabler of an effective PQS in ICH Q10 [4]. Further, while the quality system requirements for managing knowledge effectively are of primary concern, there are substantial and compelling benefits of effective KM beyond the quality system, including improved business performance 2 (see Section 2.2) and employee engagement (discussed in Section 3.5).
2.1 Knowledge Management and the Pharmaceutical Quality System
2.1 知识管理与药品质量体系
2.1.1 Introduction
2.1.1 概述
Figure 2.1, adapted from ICH Q10 [4], shows the PQS elements with the key enablers KM and QRM employed throughout the product lifecycle.
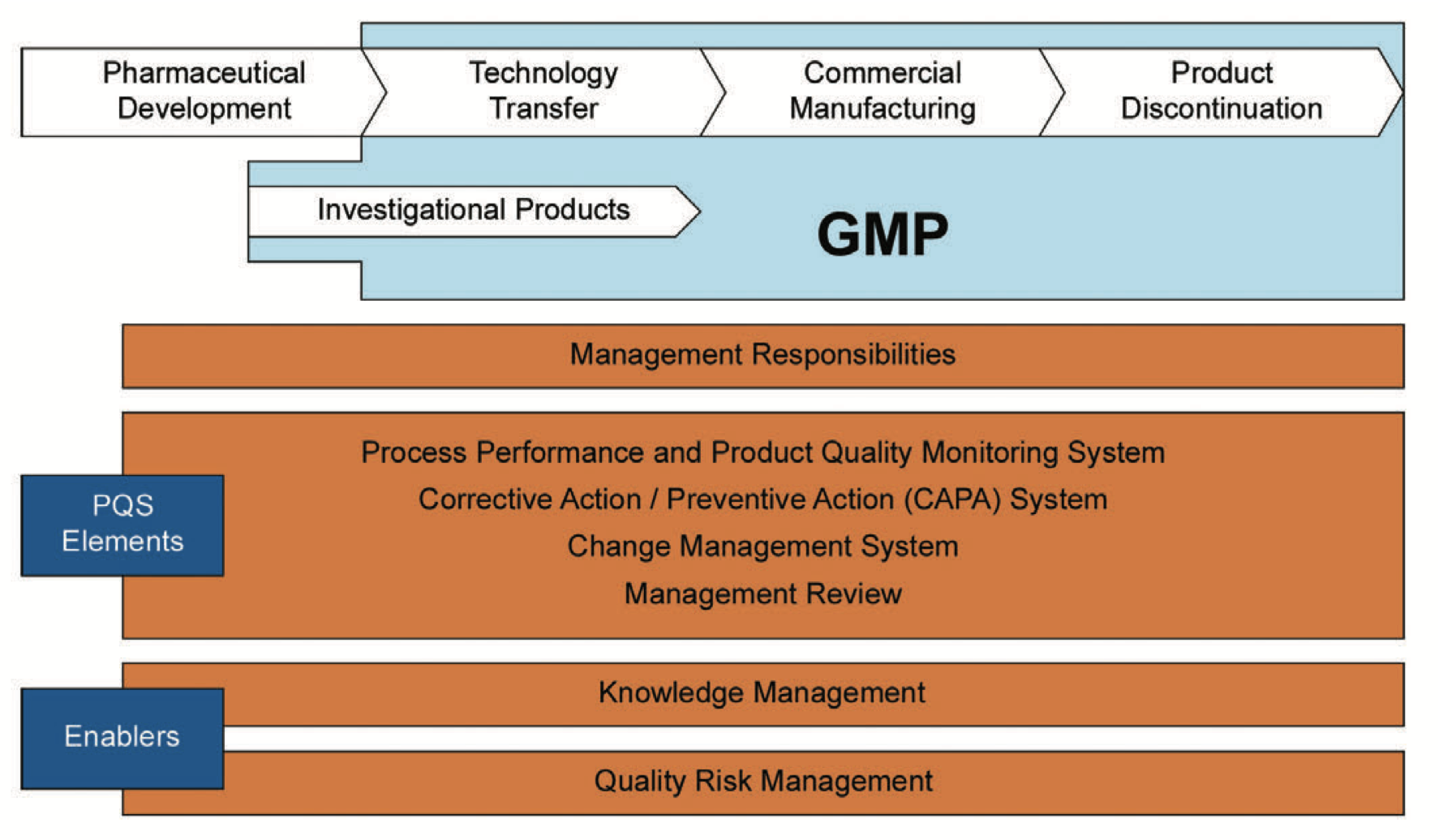
ICH Q10 [4] states:
“...knowledge management and quality risk management will enable a company to implement ICH Q10 effectively and successfully,” [thereby facilitating the achievement of the ICH Q10 objectives] “by providing the means for science and risk based decisions related to product quality.”
In this context, appropriately managing knowledge is important. ICH Q10 continues with:
“Product and process knowledge should be managed from development through the commercial life of the product up to and including product discontinuation.”
As shown in Chapter 1, ICH Q10 [4] defines Knowledge Management as:
“...a systematic approach to acquiring, analysing, storing and disseminating information related to products, manufacturing processes and components.”
A wide variety of sources of knowledge are outlined in ICH Q10 (see Section 1.7.1 of this ISPE Guide) that cover the lifecycle of a product and beyond. ICH Q11 [11], adopted in May 2012, includes references to other key sources of knowledge such as those associated with process development activities. It draws particular attention to the importance of considering knowledge transfer during technology transfer activities to internal network sites or externally to contract manufacturing partners. ICH Q11 states:
“The knowledge and process understanding should be shared as needed to perform the manufacturing process and implement the control strategy across sites involved in manufacturing the drug substance.”
Most recently, ICH Q12 [12], adopted in November 2019, describes the connection between KM and the change management process, stating that:
“An effective change management system includes active knowledge management, in which information from multiple sources is integrated to identify stimuli for changes needed to improve product and/or process robustness.”
ICH Quality Implementation Working Group on Q8, Q9, and Q10 Questions & Answers [13] and ICH Q12 both note that the need to actively manage knowledge does not confer any additional regulatory requirement to implement a formal KM system [12].
A key aspect of this chapter is how to operationalize the management of knowledge central to the overall PQS. An understanding of some of the key concepts of KM, and the systematic processes by which knowledge is managed, is therefore essential for all who work in any capacity across the pharmaceutical product lifecycle. Figure 2.2 provides a visual mapping of the relationship of KM to each component of the PQS as presented in the next several sections of this Guide.
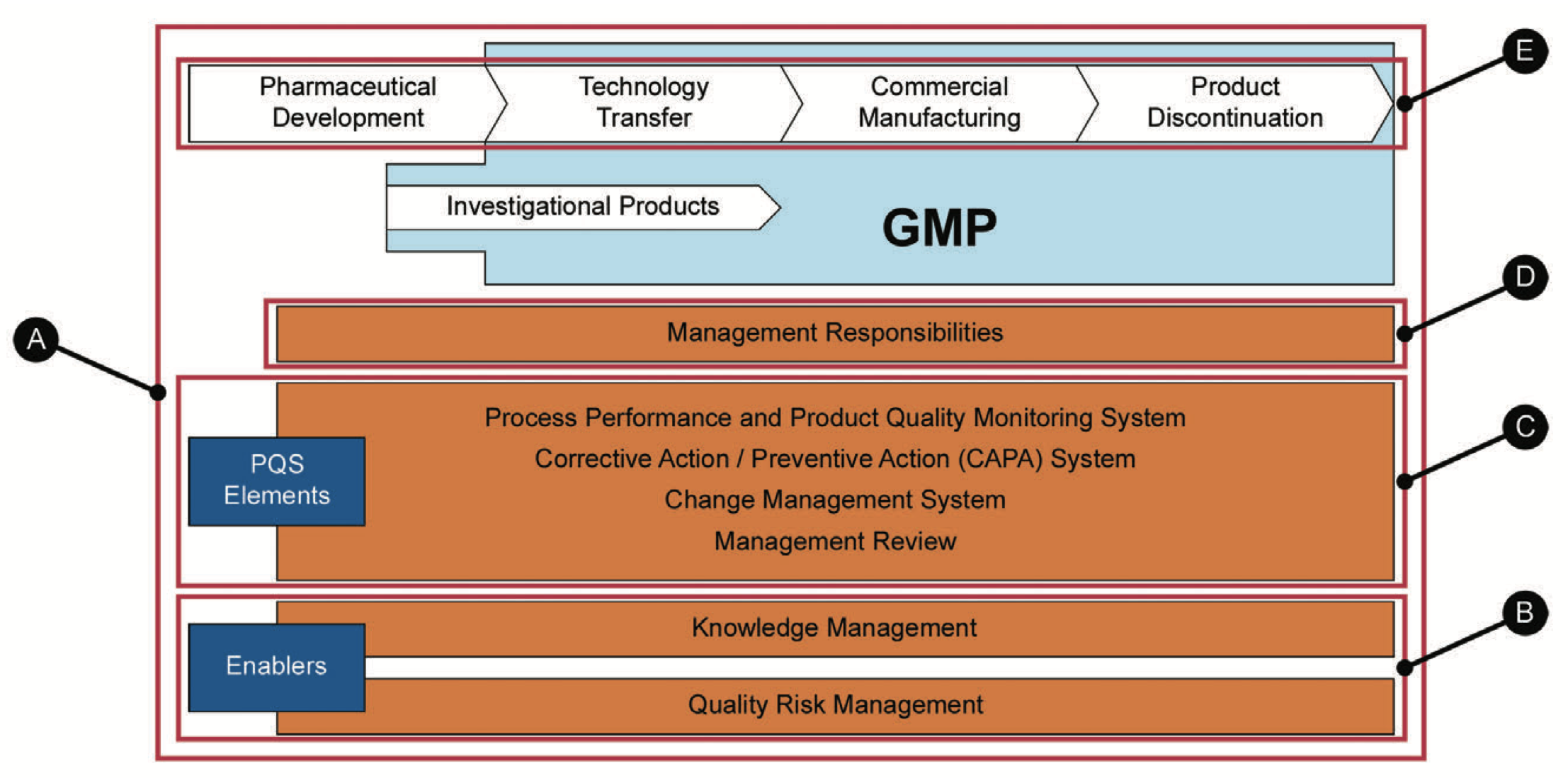
Ⓐ Section 2.1.2 focuses on how KM enables the overall objectives of an effective PQS
Ⓑ Section 2.1.3 discusses how KM can be united with QRM to enable more effective, risk-based decision-making in support of a more effective PQS
Ⓒ Section 2.1.4 highlights how KM enables each of the four major PQS elements
Ⓓ Section 2.1.5 outlines basic management responsibilities for an effective KM program
Ⓔ Section 2.1.6 introduces opportunities for KM across the product lifecycle
2.1.2 Knowledge Management in Support of ICH Q10 Objectives
2.1.2 知识管理赋能实现 ICH Q10 目标
The three objectives of ICH Q10 [4]:
- Achieve product realization
- Establish and maintain a state of control
- Facilitate continual improvement
can be better achieved using KM and QRM. Additionally, the ICH Quality Implementation Working Group on Q8, Q9 and Q10 Questions & Answers [13] clarifies that:
“Knowledge Management is not a new concept. It is always important regardless of the development approach. Q10 highlights knowledge management because it is expected that more complex information generated by appropriate approaches (e.g., QbD, PAT, real-time data generation and control monitoring systems) will need to be better captured, managed and shared during product life-cycle.
“In conjunction with Quality Risk Management, Knowledge Management can facilitate the use of concepts such as prior knowledge (including from other similar products), development of design space, control strategy, technology transfer, and continual improvement across the product life cycle.”
- 成功实现产品
- 建立并维持控制状态
- 促进持续改进
How an organization might use KM to enable these three objectives is the subject of Appendix 4, and specific KM approaches that can help facilitate the three objectives (including the use of prior knowledge, technology transfer, and continual improvement) can be found in Appendix 6. In addition, a KM program should be in place to support and enable the capture and reuse of knowledge to enable the objectives of ICH Q10 [4] and the principles included in ICH Quality Guidelines [1].
2.1.3 Knowledge Management and Quality Risk Management as the Dual Enablers of an Effective Pharmaceutical Quality System
2.1.3 知识管理和质量风险管理是有效药品质量体系的双重推动力
Effectively operating KM requires thoughtful integration with QRM [4]. These dual enablers need to function together so that the evolving knowledge base is readily available as a key input into the quality risk-based decisions that drive actions to maintain a state of control and facilitate continual improvement. Risk and knowledge (when applied) are inversely proportional. That is, increased knowledge leads to decreased uncertainty and therefore decreased risk. This concept is illustrated in Figure 2.3. Over time, as knowledge increases, risk decreases [14].

The expectation for applying scientific knowledge, for using experienced people, and for ensuring appropriate expertise is used for risk assessments is repeatedly emphasized in regulatory guidance documents. However, this knowledge must be recognized, available, and applied to be of benefit. Further, the output of the QRM process is in itself knowledge, and how this is captured, assessed, and positioned as prior knowledge is also an important consideration.
While the practice of QRM is systematic in many pharmaceutical organizations, the practice of KM still lags, and QRM and KM are not well integrated [6, 7]. Many companies conduct QRM and KM as discrete disciplines with discrete tools, approaches, and functional responsibilities. To be fully effective, the PQS needs to be configured around the integration of KM and QRM, harnessing the expertise and processes of the respective disciplines.
One pragmatic way this can be achieved in part is by instituting regular cross-functional product-level review meetings as a central part of the PQS. The outcome of such meetings should include a documentary record of the continually evolving knowledge about the product and its associated processes. This evolving knowledge should be in a format that supports effective QRM, change management, and Corrective Action and Preventive Action (CAPA), enabling the ongoing continual improvement of products, processes, and the PQS itself. This topic is further explored in Appendix 5.
The world of pharmaceutical development and manufacture is growing more complex as it moves beyond traditional manufacturing processes. Considering emerging trends such as advanced therapy medicinal products (e.g., cell and gene therapies), the use of big data and Artificial Intelligence (AI), and other trends to follow, an integrated knowledge and quality risk-centered approach will become an essential element in effectively managing product quality on an ongoing basis. An integrated approach will also support the acceleration of product launch timelines as part of routine business, getting breakthrough therapies to patients faster, or in response to a pandemic by enabling more streamlined decisions grounded in strong science, recognition of control strategy risks, and rapid regulatory submissions.
2.1.4 Knowledge Management as an Enabler to the Four Major PQS Elements
2.1.4 知识管理推动 PQS 四大要素
KM, or systematically managing knowledge, is an enabler to the four PQS elements described in ICH Q10 [4]:
- “Process performance and product quality monitoring system;
- Corrective action/preventive action [italics in original] (CAPA) system;
- Change management system;
- Management review of process performance and product quality.”
- “工艺性能和产品质量监控系统
- 纠正措施/预防措施(CAPA)系统
- 变更管理系统
- 对工艺性能和产品质量进行管理回顾”
Each of these PQS elements requires inputs (data, information, and/or knowledge) and a systematic approach to use these inputs to make decisions related to the process and the product. There are many mechanisms to collect data and information necessary to make decisions. In addition, KM methods and tools (see Appendix 6) can help make such information visible and, in conjunction with QRM tools and processes, capture tacit knowledge that may also facilitate the four PQS elements.
2.1.5 Management Responsibility for Knowledge Management
2.1.5 知识管理的管理层职责
Management has a responsibility to understand the requirements for effective KM and its role in the PQS. At the most basic level, this responsibility ensures the existence and relevance of KM efforts as a PQS enabler. These responsibilities are presented elsewhere in this Guide, including the need for KM-dedicated roles and ensuring appropriate sponsorship and governance for a KM program. (See Appendix 7.)
2.1.6 Knowledge Management across the Pharmaceutical Product Lifecycle
2.1.6 贯穿药品全生命周期的知识管理
To effectively manage pharmaceutical product knowledge, it is important to adopt an End-to-End (E2E) view of product technical knowledge, that is, across the product lifecycle. This requires evolving the traditional product lifecycle view to a product knowledge lifecycle, which enables integration of product lifecycle and platform knowledge.
Addressing this concept includes an overview of the respective KM tools, KM processes, and KM roles that can be applied to best utilize knowledge across the pharmaceutical product lifecycle. Many organizations have a collection of legacy processes and systems (IT and business) operating across this lifecycle and phases may span multiple internal and external organizations, geographies, and business processes. It can therefore be challenging to holistically identify sources of knowledge and to surface knowledge across the lifecycle. This topic is explored in more detail in Chapter 5.