ICH质量风险管理指南Q9(中英对照)
2024-01-19 27
1. Introduction
1.导言
Risk management principles are effectively utilized in many areas of business and government including finance, insurance, occupational safety, public health, pharmacovigilance, and by agencies regulating these industries. Although there are some examples of the use of quality risk management in the pharmaceutical industry today, they are limited and do not represent the full contributions that risk management has to offer. In addition, the importance of quality systems has been recognized in the pharmaceutical industry and it is becoming evident that quality risk management is a valuable component of an effective quality system.
It is commonly understood that risk is defined as the combination of the probability of occurrence of harm and the severity of that harm. However, achieving a shared understanding of the application of risk management among diverse stakeholders is difficult because each stakeholder might perceive different potential harms, place a different probability on each harm occurring and attribute different severities to each harm. In relation to pharmaceuticals, although there are a variety of stakeholders, including patients and medical practitioners as well as government and industry, the protection of the patient by managing the risk to quality should be considered of prime importance.
The manufacturing and use of a drug (medicinal) product, including its components, necessarily entail some degree of risk. The risk to its quality is just one component of the overall risk. It is important to understand that product quality should be maintained throughout the product lifecycle such that the attributes that are important to the quality of the drug (medicinal) product remain consistent with those used in the clinical studies. An effective quality risk management approach can further ensure the high quality of the drug (medicinal) product to the patient by providing a proactive means to identify and control potential quality issues during development and manufacturing. Additionally, use of quality risk management can improve the decision making if a quality problem arises. Effective quality risk management can facilitate better and more informed decisions, can provide regulators with greater assurance of a company’s ability to deal with potential risks and can beneficially affect the extent and level of direct regulatory oversight.
The purpose of this document is to offer a systematic approach to quality risk management. It serves as a foundation or resource document that is independent of, yet supports, other ICH Quality documents and complements existing quality practices, requirements, standards, and guidelines within the pharmaceutical industry and regulatory environment. It specifically provides guidance on the principles and some of the tools of quality risk management that can enable more effective and consistent risk based decisions, both by regulators and industry, regarding the quality of drug substances and drug (medicinal) products across the product lifecycle. It is not intended to create any new expectations beyond the current regulatory requirements.
It is neither always appropriate nor always necessary to use a formal risk management process (using recognized tools and/ or internal procedures e.g., standard operating procedures). The use of informal risk management processes (using empirical tools and/ or internal procedures) can also be considered acceptable. Appropriate use of quality risk management can facilitate but does not obviate industry’s obligation to comply with regulatory requirements and does not replace appropriate communications between industry and regulators.
2. Scope
2.范围
This guideline provides principles and examples of tools for quality risk management that can be applied to different aspects of pharmaceutical quality. These aspects include development, manufacturing, distribution, and the inspection and submission/review processes throughout the lifecycle of drug substances, drug (medicinal) products, biological and biotechnological products (including the use of raw materials, solvents, excipients, packaging and labeling materials in drug (medicinal) products, biological and biotechnological products).
3. Principles of quality risk management
3. 质量风险管理的原则
Two primary principles of quality risk management are:
• The evaluation of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient.(Note: Risk to quality includes situations where product availability may be impacted, leading to potential patient harm.)
• The level of effort, formality and documentation of the quality risk management process should be commensurate with the level of risk.
- 质量风险的评价应当基于科学知识,最终目的是保护患者(注:质量风险包括产品可及性可能受到影响,导致潜在患者伤害的情况);
- 质量风险管理实施过程的深度、正式程度和文件化程度都应当与风险水平相适应。
4. General quality risk management process
4.常规质量风险管理程序
Quality risk management is a systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across the product lifecycle. A model for quality risk management is outlined in the diagram (Figure 1). Other models could be used. The emphasis on each component of the framework might differ from case to case but a robust process will incorporate consideration of all the elements at a level of detail that is commensurate with the specific risk.
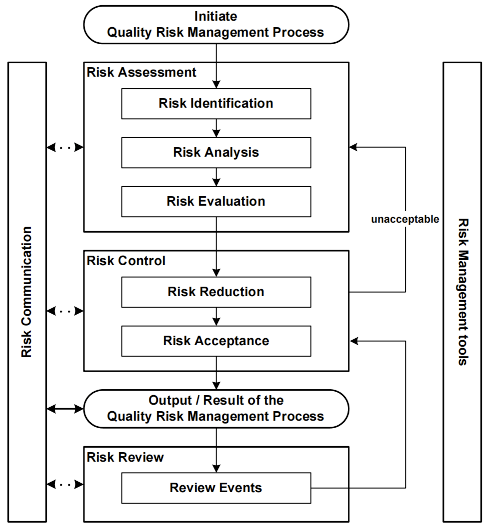
Decision nodes are not shown in the diagram above because decisions can occur at any point in the process. These decisions might be to return to the previous step and seek further information, to adjust the risk models or even to terminate the risk management process based upon information that supports such a decision. Note: “unacceptable” in the flowchart does not only refer to statutory, legislative or regulatory requirements, but also to the need to revisit the risk assessment process.
4.1. Responsibilities
4.1.职责
Quality risk management activities are usually, but not always, undertaken by interdisciplinary teams. When teams are formed, they should include experts from the appropriate areas (e.g., quality unit, business development, engineering, regulatory affairs, production operations, sales and marketing, legal, statistics and clinical) in addition to individuals who are knowledgeable about the quality risk management process.
Decision makers should
• take responsibility for coordinating quality risk management across various functions and departments of their organization;
• assure that a quality risk management process is defined, deployed and reviewed and that adequate resources are available;
and
• assure that subjectivity in quality risk management activities is managed and minimized, to facilitate scientifically robust risk-based decision-making.
- 负责协调组织内各职能部门的质量风险管理;
- 确保质量风险管理流程已经制定、实施与回顾,并配置有足够的资源和知识用。
- 确保质量风险管理活动的主观性得到管理并最大程度地降低,以促进基于风的决策更加科学稳健。
4.2. Initiating a quality risk management process
4.2.启动质量风险管理过程
Quality risk management should include systematic processes designed to coordinate, facilitate and improve science-based decision making with respect to risk. Possible steps used to initiate and plan a quality risk management process might include the following:
• Define the problem and/or risk question, including pertinent assumptions identifying the potential for risk;
• Assemble background information and/ or data on the potential hazard, harm or human health impact relevant to the risk assessment;
• Identify a leader and necessary resources;
• Specify a timeline, deliverables and appropriate level of decision making for the risk management process.
- 明确问题和/或风险疑问,包括识别潜在风险的相关假设;
- 收集与风险评估相关的潜在危害源、危害或影响人类健康的背景信息和/或数据;
- 确定负责人和必要的资源;
- 规定风险管理流程的时限、交付成果和适当的决策层级。
4.3. Risk assessment
4.3.风险评估
Risk assessment consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards (as defined below). Quality risk assessments begin with a well-defined problem description or risk question. When the risk in question is well defined, an appropriate risk management tool (see examples in section 5) and the types of information needed to address the risk question will be more readily identifiable. As an aid to clearly defining the risk(s) for risk assessment purposes, three fundamental questions are often helpful:
[1] What might go wrong?
[2] What is the likelihood (probability) it will go wrong?
[3] What are the consequences (severity)?
- 什么可能出错?
- 出错的可能有多大(可能性)?
- 后果是什么(严重性)?
Risk identification is a systematic use of information to identify hazards referring to the risk question or problem description. Information can include historical data, theoretical analysis, informed opinions, and the concerns of stakeholders. Risk identification addresses the “What might go wrong?” question, including identifying the possible consequences. This provides the basis for further steps in the quality risk management process.
Risk analysis is the estimation of the risk associated with the identified hazards. It is the qualitative or quantitative process of linking the likelihood of occurrence and severity of harms. In some risk management tools, the ability to detect the harm (detectability) also factors in the estimation of risk.
Risk evaluation compares the identified and analyzed risk against given risk criteria. Risk evaluations consider the strength of evidence for all three of the fundamental questions.
In doing an effective risk assessment, the robustness of the data set is important because it determines the quality of the output. Revealing assumptions and reasonable sources of uncertainty will enhance confidence in this output and/or help identify its limitations. Uncertainty is due to combination of incomplete knowledge about a process and its expected or unexpected variability. Typical sources of uncertainty include gaps in knowledge gaps in pharmaceutical science and process understanding, sources of harm (e.g., failure modes of a process, sources of variability), and probability of detection of problems.
The output of a risk assessment is either a quantitative estimate of risk or a qualitative description of a range of risk. When risk is expressed quantitatively, a numerical probability is used. Alternatively, risk can be expressed using qualitative descriptors, such as “high”, “medium”, or “low”, which should be defined in as much detail as possible. Sometimes a "risk score" is used to further define descriptors in risk ranking. In quantitative risk assessments, a risk estimate provides the likelihood of a specific consequence, given a set of risk-generating circumstances. Thus, quantitative risk estimation is useful for one particular consequence at a time. Alternatively, some risk management tools use a relative risk measure to combine multiple levels of severity and probability into an overall estimate of relative risk. The intermediate steps within a scoring process can sometimes employ quantitative risk estimation.
4.4. Risk control
4.4.风险控制
Risk control includes decision making to reduce and/or accept risks. The purpose of risk control is to reduce the risk to an acceptable level. The amount of effort used for risk control should be proportional to the significance of the risk. Decision makers might use different processes, including benefit-cost analysis, for understanding the optimal level of risk control.
Risk control might focus on the following questions:
• Is the risk above an acceptable level?
• What can be done to reduce or eliminate risks?
• What is the appropriate balance among benefits, risks and resources?
• Are new risks introduced as a result of the identified risks being controlled?
- 风险是否高于可接受水平?
- 什么措施可以用来降低或消除风险?
- 什么是利益、风险和资源间合适的平衡点?
- 控制所识别风险时是否会引入新的风险?
Risk reduction focuses on processes for mitigation or avoidance of quality risk when it exceeds a specified (acceptable) level (see Fig. 1). Risk reduction might include actions taken to mitigate the severity and probability of harm. Processes that improve the detectability of hazards and quality risks might also be used as part of a risk control strategy. The implementation of risk reduction measures can introduce new risks into the system or increase the significance of other existing risks. Hence, it might be appropriate to revisit the risk assessment to identify and evaluate any possible change in risk after implementing a risk reduction process.
Risk acceptance is a decision to accept risk. Risk acceptance can be a formal decision to accept the residual risk or it can be a passive decision in which residual risks are not specified. For some types of harms, even the best quality risk management practices might not entirely eliminate risk. In these circumstances, it might be agreed that an appropriate quality risk management strategy has been applied and that quality risk is reduced to a specified (acceptable) level. This (specified) acceptable level will depend on many parameters and should be decided on a case-by-case basis.
4.5. Risk communication
4.5.风险沟通
Risk communication is the sharing of information about risk and risk management between the decision makers and others. Parties can communicate at any stage of the risk management process (see Fig. 1: dashed arrows). The output/result of the quality risk management process should be appropriately communicated and documented (see Fig. 1: solid arrows). Communications might include those among interested parties; e.g., regulators and industry, industry and the patient, within a company, industry or regulatory authority, etc. The included information might relate to the existence, nature, form, probability, severity, acceptability, control, treatment, detectability or other aspects of risks to quality. Communication need not be carried out for each and every risk acceptance. Between the industry and regulatory authorities, communication concerning quality risk management decisions might be effected through existing channels as specified in regulations and guidances.
4.6. Risk review
4.6.风险评审
Risk management should be an ongoing part of the quality management process. A mechanism to review or monitor events should be implemented.
The output/results of the risk management process should be reviewed to take into account new knowledge and experience. Once a quality risk management process has been initiated, that process should continue to be utilized for events that might impact the original quality risk management decision, whether these events are planned (e.g., results of product review, inspections, audits, change control) or unplanned (e.g., root cause from failure investigations, recall). The frequency of any review should be based upon the level of risk. Risk review might include reconsideration of risk acceptance decisions (section 4.4).
5. Risk management methodology
5.风险管理方法学
Quality risk management supports a scientific and practical approach to decision-making. It provides documented, transparent and reproducible methods to accomplish steps of the quality risk management process based on current knowledge about assessing the probability, severity and sometimes detectability of the risk.
Traditionally, risks to quality have been assessed and managed in a variety of informal ways (empirical and/ or internal procedures) based on, for example, compilation of observations, trends and other information. Such approaches continue to provide useful information that might support topics such as handling of complaints, quality defects, deviations and allocation of resources.
Additionally, the pharmaceutical industry and regulators can assess and manage risk using recognized risk management tools and/ or internal procedures (e.g., standard operating procedures). Below is a non-exhaustive list of some of these tools (further details in Annex 1 and chapter 8):
•Basic risk management facilitation methods
(flowcharts, check sheets etc.);
•Failure Mode Effects Analysis (FMEA);
• Failure Mode, Effects and Criticality Analysis (FMECA);
•Fault Tree Analysis (FTA);
• Hazard Analysis and Critical Control Points (HACCP);
•Hazard Operability Analysis (HAZOP);
•Preliminary Hazard Analysis (PHA);
•Risk ranking and filtering;
• Supporting statistical tools.
- 基本风险管理引导方法(流程图,检查表等);
- 失效模式与影响分析(FMEA);
- 失效模式、影响与关键性分析(FMECA);
- 故障树分析(FTA);
- 危害源分析及关键控制点(HACCP);
- 危害源及可操作性分析(HAZOP);
- 初步危害源分析(PHA);
- 风险排序及筛选;
- 支持性统计工具。
It might be appropriate to adapt these tools for use in specific areas pertaining to drug substance and drug (medicinal) product quality. Quality risk management methods and the supporting statistical tools can be used in combination (e.g., Probabilistic Risk Assessment). Combined use provides flexibility that can facilitate the application of quality risk management principles.
The degree of rigor and formality of quality risk management should reflect available knowledge and be commensurate with the complexity and/ or criticality of the issue to be addressed.
5.1 Formality in Quality Risk Management
5.1 质量风险管理的正式性
Formality in quality risk management is not a binary concept (i.e. formal/informal); varying degrees of formality may be applied during quality risk management activities, including when making risk-based decisions. In this way, formality can be considered a continuum (or spectrum), ranging from low to high.
When determining how much formality to apply to a given quality risk management activity, certain factors may be considered. These may include, for example, the following:
• Uncertainty: The term “uncertainty” in quality risk management means lack of knowledge about hazards, harms and, consequently, their associated risks. The level of uncertainty that is associated with the area being risk assessed informs how much formality may be required to manage potential risks. Systematic approaches for acquiring, analysing, storing and disseminating scientific information are essential for generating knowledge, which in turn informs all quality risk management activities. Uncertainty may be reduced via effective knowledge management, which enables accumulated and new information (both internal and external) to be used to support risk-based decisions throughout the product lifecycle.
- 不确定性:质量风险管理中的“不确定性”意味着对相关危害源、危害、及其所致风险 了解较少。风险评估范围相关的不确定性水平显示了管理潜在风险可能需要的正式程度的高低。系统地获取、分析、储存和传播科学信息的方法对于产生知识至关重要,而知识反过来又为所有质量风险管理活动提供信息。通过有效的知识管理,可以减少不确定性,从而在整个产品生命周期中使用积累的和新的信息(内部和外部)来支持基于风险的决策。
• Importance: The more important a risk-based decision may be in relation to product quality, the higher the level of formality that should be applied, and the greater the need to reduce the level of uncertainty associated with it.
- 重要性:基于风险的决策对产品质量越重要,采用的正式程度应越高,就更需要减少与其相关的不确定性水平。
• Complexity: The more complex a process or subject area is to a quality risk management activity, the higher the level of formality that should be applied to assure product quality
- 复杂性:为确保产品质量,质量风险管理活动的过程或专业领域越复杂,,应采用的正式程度就越高。
Higher levels of uncertainty, importance or complexity may require more formal quality risk management approaches to manage potential risks and to support effective risk-based decision making
The overall approach for determining how much formality to apply during quality risk management activities should be described within the quality system. Resource constraints should not be used to justify the use of lower levels of formality in the quality risk management process. Risk scores, ratings and assessments should be based on an appropriate use of evidence, science and knowledge. Regardless of how much formality is applied, the robust management of risk is the goal of the process
The following may be characteristics of higher levels of formality:
• All parts of the quality risk management process (risk assessment, risk control, risk review and risk communication) are explicitly performed, and stand-alone quality risk management reports or related documents which address all aspects of the process may be generated and are documented (e.g., within the quality system).
• Quality risk management tools, including those shown in Annex 1, are used in some or all parts of the process.
• A cross-functional team is assembled for the quality risk management activity.
• Use of a facilitator, with experience and knowledge of the quality risk management process, may be integral to a higher formality process.
- 明确执行质量风险管理过程的所有步骤(风险评估、风险控制、风险回顾和风险沟 通),可形成独立的质量风险管理报告或相关文件,将过程中所有相关活动予以书面记录 (例如,在质量体系内)。
- 在过程的某些或所有部分使用质量风险管理工具,包括附录 1 中所示工具。
- 为质量风险管理活动组建一个跨部门的团队。
- 使用具有质量风险管理流程方面的经验和知识的引导者可能是较高正式程度的流程所不可或缺的。
The following may be characteristics of lower levels of formality:
• One or more parts of the quality risk management process are not performed as standalone activities but are addressed within other elements of the quality system which may have risk assessment and risk control activities embedded within them.
• Quality risk management tools might not be used in some or all parts of the process.
• A cross-functional team might not be necessary.
• Stand-alone quality risk management reports might not be generated. The outcome of the quality risk management process is usually documented in the relevant parts of the quality system.
- 一个或多个部分的质量风险管理过程不是作为独立活动执行的,而是在质量体系的其他要素中处理的,可能包含风险评估和风险控制活动。
- 质量风险管理工具可能不会在质量风险管理的某些或全部流程中使用。
- 可能不需要跨部门团队。
- 质量风险管理过程的结果通常记录在质量体系的相关部分中,可能不会生成独立的质 量风险管理报告。
5.2 Risk-Based Decision-Making
5.2 基于风险的决策
Risk-based decision-making is inherent in all quality risk management activities; it provides an essential foundation for decision makers in an organization. Effective risk-based decisionmaking begins with determining the level of effort, formality and documentation that should be applied during the quality risk management process. The decisions made from quality risk management activities include those in relation to what hazards exist, the risks associated with those hazards, the risk controls required, the acceptability of the residual risk after risk controls, and also the communication and review of quality risk management activities and outputs.
As all decision-making relies on the use of knowledge, see ICH Q10 for guidance in relation to knowledge management. It is important also to ensure the integrity of the data that are used for risk-based decision-making.
There are different processes that may be used to make risk-based decisions; these are directly related to the level of formality that is applied during the quality risk management process. (See Section 5.1 above for guidance on what constitutes formality in quality risk management.)
Higher levels of formality in quality risk management may require higher levels of structure in relation to risk-based decision-making. There can be varying degrees of structure with regard to approaches for risk-based decision-making. These degrees of structure can be considered to be on a continuum (or spectrum). Below are descriptions of highly structured vs. less structured processes, and for rule-based processes when making risk-based decisions:
• Some risk-based decision-making processes are highly structured and can involve a formal analysis of the available options that exist before making a decision. They involve an indepth consideration of relevant factors associated with the available options. Such processes might be used when there is a high degree of importance associated with the decision, and when the level of uncertainty and/or complexity is high.
- 一些基于风险的决策过程是高度结构化的,在做出决策之前,可以对现有的可用选项 进行正式分析,涉及对与可用选项相关因素的深入考虑。当决策非常重要,同时不确定性和/或复杂性水平较高时,可使用此类流程。
• Other risk-based decision-making processes are less structured; here, simpler approaches are used to arrive at decisions, and they primarily make use of existing knowledge to support an assessment of hazards, risks and any required risk controls. Such processes might still be used when there is a high degree of importance associated with the decision, but the degree of uncertainty and/or complexity is lower.
- 另一些基于风险的决策过程是较低结构化的流程,使用更简单的方法做出决策,它们主要利用现有知识支持对危害源、风险和任何必要的风险控制进行评估。当决策非常重要,但不确定性和/或复杂性较低时,仍可使用此类过程。
• Decisions might also be made using rule-based (or standardized) approaches, which do not require a new risk assessment to make such decisions. This is where there are SOPs, policies or well understood requirements in place which determine what decisions must be made. Here, rules (or limits) may be in place which govern such decisions; these may be based on a previously obtained understanding of the relevant risks and they usually lead to predetermined actions and/or expected outcomes
- 也可以使用基于规则(或标准化)的方法做出决策,这些方法不需要新的风险评估来做出此类决策,即在有 SOP、政策或充分理解需求的地方,就已经决定了必须做出何种决策。这时,可能要有规则(或限制)来管理这些决策,因为这些决定可能是基于以往对相关风险 的了解做出的,通常会指向预定的行动和/或预期结果。
The above approaches to risk-based decision-making are beneficial because they address uncertainty through the use of knowledge, facilitating informed decisions by regulators and the pharmaceutical industry in a multitude of areas. They also help recognize where uncertainty remains, so that appropriate risk controls (including improved detection) may be identified to enhance understanding of those variables and further reduce the level of uncertainty.
5.3 Managing and Minimizing Subjectivity
5.3 管理并最大程度地降低主观性
Subjectivity can impact every stage of a quality risk management process, especially the identification of hazards and the estimation of probability of occurrence and severity of harm. It can also impact the estimation of risk reduction and the effectiveness of decisions made from quality risk management activities.
Subjectivity can be introduced in quality risk management through differences in how risks are assessed and in how hazards, harms and risks are perceived by different stakeholders, (e.g., bias). Subjectivity can also be introduced when risk questions are inadequately defined, and when tools have poorly designed risk scoring scales.
While subjectivity cannot be completely eliminated from quality risk management activities, it may be controlled by addressing bias and assumptions, the proper use of quality risk management tools and maximizing the use of relevant data and sources of knowledge (see ICH Q10, Section 1.6.1).
All participants involved with quality risk management activities should acknowledge, anticipate, and address the potential for subjectivity.
6. Integration of quality risk management into industry and regulatory operations
6.质量风险管理与业界及药政运行整合
Quality risk management is a process that supports science-based and practical decisions when integrated into quality systems (see Annex II). As outlined in the introduction, appropriate use of quality risk management does not obviate industry’s obligation to comply with regulatory requirements. However, effective quality risk management can facilitate better and more informed decisions, can provide regulators with greater assurance of a company’s ability to deal with potential risks, and might affect the extent and level of direct regulatory oversight. In addition, quality risk management can facilitate better use of resources by all parties.
Training of both industry and regulatory personnel in quality risk management processes provides for greater understanding of decision-making processes and builds confidence in quality risk management outcomes.
Quality risk management should be integrated into existing operations and documented appropriately. Annex II provides examples of situations in which the use of the quality risk management process might provide information that could then be used in a variety of pharmaceutical operations. These examples are provided for illustrative purposes only and should not be considered a definitive or exhaustive list. These examples are not intended to create any new expectations beyond the requirements laid out in the current regulations.
Examples for industry and regulatory operations (see Annex II):
Quality management.
- 质量管理。
Examples for industry operations and activities (see Annex II):
• Development;
• Facility, equipment and utilities;
• Materials management;
• Production;
• Laboratory control and stability testing;
• Packaging and labeling.
• Supply chain control.
- 研发;
- 厂房、设备和设施;
- 物料管理;
- 生产;
- 实验室控制和稳定性试验;
- 包装和贴签。
- 供应链控制。
Examples for regulatory operations (see Annex II):
• Inspection and assessment activities.
- 检查与评估工作。
While regulatory decisions will continue to be taken on a regional basis, a common understanding and application of quality risk management principles could facilitate mutual confidence and promote more consistent decisions among regulators on the basis of the same information. This collaboration could be important in the development of policies and guidelines that integrate and support quality risk management practices.
6.1 The role of Quality Risk Management in Addressing Product Availability Risks Arising from Quality/Manufacturing Issues
6.1 质量风险管理在应对因质量/生产问题引起产品可及性风险中的作用
Quality/manufacturing issues, including non-compliance with Good Manufacturing Practice (GMP), are a significant cause of product availability issues (e.g., product shortages). The interests of patients are served by risk-based drug shortage prevention and mitigation activities that help to proactively manage supply chain complexities and ensure availability of needed drug (medicinal) products.
While manufacturing and supply chain diversity can be enablers of product availability, increasingly complex supply chains lead to interdependencies that can introduce systemic quality/manufacturing risks impacting supply chain robustness. The application of quality risk management enables the proactive identification and implementation of preventive measures that support product availability.
An effective pharmaceutical quality system drives both supply chain robustness and sustainable GMP compliance. The pharmaceutical quality system, including management responsibilities, also uses quality risk management and knowledge management to provide an early warning system that supports effective oversight and response to evolving quality/manufacturing risks from the pharmaceutical company or its external partners. When risk-based drug shortage prevention and mitigation activities are performed, the level of formality that is applied to those activities may vary (see Section 5.1) and should be commensurate with the level of risk associated with a loss of availability of the product(s).
Quality/manufacturing factors that can affect supply reliability, and hence product availability, include, but are not limited to, the following:
Processes that exhibit excessive variability (e.g., process drift, non-uniformity) have capability gaps that can result in unpredictable outputs (e.g., quality, timeliness and yield) and consequently can adversely impact product availability. Quality risk management can help design monitoring systems that are capable of detecting departures from a state of control and deficiencies in manufacturing processes, so they can be investigated to address root causes.
变异性(例如工艺漂移、非均一)过高的工艺存在能力缺陷,这种缺陷可导致不可预测的结果(例如质量、时限性和收率),并可能会给产品可及性带来不良影响。质量风险管理可帮助设计出能够检测到受控状态偏离和生产工艺缺陷的监测系统,这样就可以对其进行调查,来明确其根本原因。
A robust facility infrastructure can facilitate reliable supply; it includes suitable equipment and well-designed facilities for manufacturing (including packaging and testing). Robustness can be affected by multiple factors, such as an aging facility, insufficient maintenance or an operational design that is vulnerable to human error. Risks to supply can be reduced by addressing these factors, as well as through the use of modern technology, such as digitalization, automation, isolation technology, amongst others.
稳固可靠的厂房基础设施能够促进可靠的供应,包括为生产(包括包装和测试)所选择适宜的设备和设计良好的厂房设施。可靠性可受多种因素的影响,包括厂房老化、维护不充分或易导致人员差错的操作设计。可通过解决这些因素,及采用如数字化、自动化、隔离技术等现代技术来降低供应风险。
Quality system governance includes assuring the acceptability of supply chain partners over the product lifecycle. Approval and oversight of outsourced activities and material suppliers is informed by risk assessments, effective knowledge management, and an effective monitoring strategy for supply chain partner performance. A successful manufacturing partnership is strengthened by appropriate communication and collaboration mechanisms (See Section 2.7 of ICH Q10). When substantial variability is identified in the quality and safety of supplied materials or in the services provided, enhanced review and monitoring activities are justified. In some cases, it may be necessary to identify a new supply chain entity (e.g., a pre-qualified alternative option) to perform a function.
质量体系管理包括在产品生命周期中确保供应链合作伙伴的可接受性。通过风险评估、 有效的知识管理和有效的供应链合作伙伴绩效监控策略,对外包活动和物料供应商进行审批和监督。适宜的沟通和合作机制可以加强成功的生产合作伙伴关系。如发现所供应物料或所提供服务的质量和安全方面存在重大的变化时,则需要加强审核和监测工作(见 ICH Q10第 2.7 部分)。在某些情况下,可能需要寻找新的供应链实体(例如经过资质确认的备选选项)来完成某项职能。
Note that the guidance in Annex II.2, in relation to the application of quality risk management as part of Regulatory Operations, can be useful to consider in the context of product availability risks.
7. Definitions
7.定义
Decision maker(s):
Person(s) with the competence and authority to make appropriate and timely quality risk management decisions.
Detectability:
The ability to discover or determine the existence, presence, or fact of a hazard.
Harm:
Damage to health, including the damage that can occur from loss of product quality or availability.
Hazard:
The potential source of harm (ISO/IEC Guide 51).
Product lifecycle:
All phases in the life of the product from the initial development through marketing until the product’s discontinuation.
Quality:
The degree to which a set of inherent properties of a product, system or process fulfills requirements (see ICH Q6A definition specifically for "quality" of drug substance and drug (medicinal) products.)
Quality risk management:
A systematic process for the assessment, control, communication and review of risks to the quality of the drug (medicinal) product across the product lifecycle.
Quality system:
The sum of all aspects of a system that implements quality policy and ensures that quality objectives are met.
Requirements:
The explicit or implicit needs or expectations of the patients or their surrogates (e.g., health care professionals, regulators and legislators). In this document, “requirements” refers not only to statutory, legislative, or regulatory requirements, but also to such needs and expectations.
Risk:
The combination of the probability of occurrence of harm and the severity of that harm (ISO/IEC Guide 51).
Risk acceptance:
The decision to accept risk (ISO Guide 73).
Risk analysis:
The estimation of the risk associated with the identified hazards.
Risk assessment:
A systematic process of organizing information to support a risk decision to be made within a risk management process. It consists of the identification of hazards and the analysis and evaluation of risks associated with exposure to those hazards.
Risk communication:
The sharing of information about risk and risk management between the decision maker and other stakeholders.
Risk control:
Actions implementing risk management decisions (ISO Guide 73).
Risk evaluation:
The comparison of the estimated risk to given risk criteria using a quantitative or qualitative scale to determine the significance of the risk.
Risk identification:
The systematic use of information to identify potential sources of harm (hazards) referring to the risk question or problem description.
Risk management:
The systematic application of quality management policies, procedures, and practices to the tasks of assessing, controlling, communicating and reviewing risk.
Risk reduction:
Actions taken to lessen the probability of occurrence of harm and the severity of that harm.
Risk review:
Review or monitoring of output/results of the risk management process considering (if appropriate) new knowledge and experience about the risk.
Severity:
A measure of the possible consequences of a hazard.
Stakeholder:
Any individual, group or organization that can affect, be affected by, or perceive itself to be affected by a risk. Decision makers might also be stakeholders. For the purposes of this guideline, the primary stakeholders are the patient, healthcare professional, regulatory authority, and industry.
Trend:
A statistical term referring to the direction or rate of change of a variable(s).
8. References
8.参考文献
Annex I: risk management methods and tools
附录I:风险管理方法与工具
Some of the simple techniques that are commonly used to structure risk management by organizing data and facilitating decision-making are:
•Flowcharts;
•Check Sheets;
• Process Mapping;
• Cause and Effect Diagrams (also called an Ishikawa diagram or fish bone diagram).
- 流程图;
- 检查表;
- 过程流程图;
- 因果图(也称为石川图或鱼骨图)
I.2 Failure Mode Effects Analysis (FMEA)
I.2故障模式效应分析(FMEA)
FMEA (see IEC 60812) provides for an evaluation of potential failure modes for processes and their likely effect on outcomes and/or product performance. Once failure modes are established, risk reduction can be used to eliminate, contain, reduce or control the potential failures. FMEA relies on product and process understanding. FMEA methodically breaks down the analysis of complex processes into manageable steps. It is a powerful tool for summarizing the important modes of failure, factors causing these failures and the likely effects of these failures.
Potential Areas of Use(s)
FMEA can be used to prioritize risks and monitor the effectiveness of risk control activities.
FMEA can be applied to equipment and facilities and might be used to analyze a manufacturing operation and its effect on product or process. It identifies elements/operations within the system that render it vulnerable. The output/ results of FMEA can be used as a basis for design or further analysis or to guide resource deployment.
I.3 Failure Mode, Effects and Criticality Analysis (FMECA)
I.3故障模式影响与严重性分析(FMECA)
FMEA might be extended to incorporate an investigation of the degree of severity of the consequences, their respective probabilities of occurrence, and their detectability, thereby becoming a Failure Mode Effect and Criticality Analysis (FMECA; see IEC 60812). In order for such an analysis to be performed, the product or process specifications should be established. FMECA can identify places where additional preventive actions might be appropriate to minimize risks.
Potential Areas of Use(s)
FMECA application in the pharmaceutical industry should mostly be utilized for failures and risks associated with manufacturing processes; however, it is not limited to this application. The output of an FMECA is a relative risk “score” for each failure mode, which is used to rank the modes on a relative risk basis.
I.4 Fault Tree Analysis (FTA)
I.4故障树分析(FTA)
The FTA tool (see IEC 61025) is an approach that assumes failure of the functionality of a product or process. This tool evaluates system (or sub-system) failures one at a time but can combine multiple causes of failure by identifying causal chains. The results are represented pictorially in the form of a tree of fault modes. At each level in the tree, combinations of fault modes are described with logical operators (AND, OR, etc.). FTA relies on the experts’ process understanding to identify causal factors.
Potential Areas of Use(s)
FTA can be used to establish the pathway to the root cause of the failure. FTA can be used to investigate complaints or deviations in order to fully understand their root cause and to ensure that intended improvements will fully resolve the issue and not lead to other issues (i.e. solve one problem yet cause a different problem). Fault Tree Analysis is an effective tool for evaluating how multiple factors affect a given issue. The output of an FTA includes a visual representation of failure modes. It is useful both for risk assessment and in developing monitoring programs.
I.5 Hazard Analysis and Critical Control Points (HACCP)
I.5危害分析关键控制点(HACCP)
HACCP is a systematic, proactive, and preventive tool for assuring product quality, reliability, and safety (see WHO Technical Report Series No 908, 2003 Annex 7). It is a structured approach that applies technical and scientific principles to analyze, evaluate, prevent, and control the risk or adverse consequence(s) of hazard(s) due to the design, development, production, and use of products.
HACCP consists of the following seven steps:
• conduct a hazard analysis and identify preventive measures for each step of the process;
• determine the critical control points;
• establish critical limits;
•establish a system to monitor the critical control points;
• establish the corrective action to be taken when monitoring indicates that the critical control points are not in a state of control;
•establish system to verify that the HACCP system is working effectively;
• establish a record-keeping system.
- 对过程的每一步进行危险分析并辨识预防措施;
- 确定关键控制点;
- 建立关键限度;
- 建立一个监测关键控制点的监控体系;
- 建立当监测显示关键控制点并不在控制状态时应该采取的纠正措施;
- 建立证实危害分析关键控制点体系在有效运转的系统;
- 建立一个保持记录的系统。
Potential Areas of Use(s)
HACCP might be used to identify and manage risks associated with physical, chemical and biological hazards (including microbiological contamination). HACCP is most useful when product and process understanding is sufficiently comprehensive to support identification of critical control points. The output of a HACCP analysis is risk management information that facilitates monitoring of critical points not only in the manufacturing process but also in other life cycle phases.
I.6 Hazard Operability Analysis (HAZOP)
I.6 危害及可操作性分析(HAZOP)
HAZOP (see IEC 61882) is based on a theory that assumes that risk events are caused by deviations from the design or operating intentions. It is a systematic brainstorming technique for identifying hazards using so-called “guide-words”. “Guide-words” (e.g., No, More, Other Than, Part of, etc.) are applied to relevant parameters (e.g., contamination, temperature) to help identify potential deviations from normal use or design intentions. It often uses a team of people with expertise covering the design of the process or product and its application.
Potential Areas of Use(s)
HAZOP can be applied to manufacturing processes, including outsourced production and formulation as well as the upstream suppliers, equipment and facilities for drug substances and drug (medicinal) products. It has also been used primarily in the pharmaceutical industry for evaluating process safety hazards. As is the case with HACCP, the output of a HAZOP analysis is a list of critical operations for risk management. This facilitates regular monitoring of critical points in the manufacturing process.
I.7 Preliminary Hazard Analysis (PHA)
I.7 预先危险分析(PHA)
PHA is a tool of analysis based on applying prior experience or knowledge of a hazard or failure to identify future hazards, hazardous situations and events that might cause harm, as well as to estimate their probability of occurrence for a given activity, facility, product or system. The tool consists of: 1) the identification of the possibilities that the risk event happens, 2) the qualitative evaluation of the extent of possible injury or damage to health that could result and 3) a relative ranking of the hazard using a combination of severity and likelihood of occurrence, and 4) the identification of possible remedial measures.
Potential Areas of Use(s)
PHA might be useful when analyzing existing systems or prioritizing hazards where circumstances prevent a more extensive technique from being used. It can be used for product, process and facility design as well as to evaluate the types of hazards for the general product type, then the product class, and finally the specific product. PHA is most commonly used early in the development of a project when there is little information on design details or operating procedures; thus, it will often be a precursor to further studies. Typically, hazards identified in the PHA are further assessed with other risk management tools such as those in this section.
I.8 Risk ranking and filtering
I.8 风险排序及过滤
Risk ranking and filtering is a tool for comparing and ranking risks. Risk ranking of complex systems typically requires evaluation of multiple diverse quantitative and qualitative factors for each risk. The tool involves breaking down a basic risk question into as many components as needed to capture factors involved in the risk. These factors are combined into a single relative risk score that can then be used for ranking risks. “Filters,” in the form of weighting factors or cut-offs for risk scores, can be used to scale or fit the risk ranking to management or policy objectives.
Potential Areas of Use(s)
Risk ranking and filtering can be used to prioritize manufacturing sites for inspection/audit by regulators or industry. Risk ranking methods are particularly helpful in situations in which the portfolio of risks and the underlying consequences to be managed are diverse and difficult to compare using a single tool. Risk ranking is useful when management needs to evaluate both quantitatively-assessed and qualitatively-assessed risks within the same organizational framework.
I.9 Supporting statistical tools
I.9 辅助性统计工具
- Control Charts, for example:Acceptance Control Charts (see ISO 7966);Control Charts with Arithmetic Average and Warning Limits (see ISO 7873);Cumulative Sum Charts (see ISO 7871);Shewhart Control Charts (see ISO 8258);Weighted Moving Average.
- Acceptance Control Charts (see ISO 7966);
- Control Charts with Arithmetic Average and Warning Limits (see ISO 7873);
- Cumulative Sum Charts (see ISO 7871);
- Shewhart Control Charts (see ISO 8258);
- Weighted Moving Average.
- Design of Experiments (DOE);
- Histograms;
- Pareto Charts;
- Process Capability Analysis.
- 控制图,例如: 验收控制图(参见ISO 7870-3:2020); 累积和图(参见ISO7870-4:2021); 休哈特控制图(参见ISO7870-2:2013); 加权移动平均控制图。
- 验收控制图(参见ISO 7870-3:2020);
- 累积和图(参见ISO7870-4:2021);
- 休哈特控制图(参见ISO7870-2:2013);
- 加权移动平均控制图。
- 实验设计(DOE);
- 直方图;
- 帕累托图;
- 过程能力分析。
Annex II: Potential applications for quality risk management
附录 II: 实施质量风险管理的潜在机会
This Annex is intended to identify potential uses of quality risk management principles and tools by industry and regulators. However, the selection of particular risk management tools is completely dependent upon specific facts and circumstances.
These examples are provided for illustrative purposes and only suggest potential uses of quality risk management. This Annex is not intended to create any new expectations beyond the current regulatory requirements.
II.1 Quality risk management as part of integrated quality management
II.1 整合质量管理部分的质量风险管理
Documentation
To review current interpretations and application of regulatory expectations;
To determine the desirability of and/or develop the content for SOPs, guidelines, etc.
Training and education
To determine the appropriateness of initial and/or ongoing training sessions based on education, experience and working habits of staff, as well as on a periodic assessment of previous training (e.g., its effectiveness);
To identify the training, experience, qualifications and physical abilities that allow personnel to perform an operation reliably and with no adverse impact on the quality of the product.
Quality defects
To provide the basis for identifying, evaluating, and communicating the potential quality impact of a suspected quality defect, complaint, trend, deviation, investigation, out of specification result, etc;
To facilitate risk communications and determine appropriate action to address significant product defects, in conjunction with regulatory authorities (e.g., recall).
Auditing/Inspection
To define the frequency and scope of audits, both internal and external, taking into account factors such as:
• Existing legal requirements;
• Overall compliance status and history of the company or facility;
• Robustness of a company’s quality risk management activities;
• Complexity of the site;
• Complexity of the manufacturing process;
• Complexity of the product and its therapeutic significance;
• Number and significance of quality defects (e.g., recall);
• Results of previous audits/inspections;
• Major changes of building, equipment, processes, key personnel;
• Experience with manufacturing of a product (e.g., frequency, volume, number of batches);
• Test results of official control laboratories.
- 现行法律要求;
- 公司或工厂整体的合规状态及历史;
- 公司质量风险管理活动的稳健性;
- 场地的复杂性;
- 生产工艺的复杂性;
- 产品的复杂性及其临床治疗意义;
- 质量缺陷(如召回)的数量及重要性;
- 以往的审计/检查结果;
- 建筑物、设备、工艺、关键人员的重大变更;
- 产品的生产经验(如频率、产量、批次);
- 官方药品检验机构的检测结果。
Periodic review
To select, evaluate and interpret trend results of data within the product quality review;
To interpret monitoring data (e.g., to support an assessment of the appropriateness of revalidation or changes in sampling).
Change management / change control
To manage changes based on knowledge and information accumulated in pharmaceutical development and during manufacturing;
To evaluate the impact of the changes on the availability of the final product;
To evaluate the impact on product quality of changes to the facility, equipment, material, manufacturing process or technical transfers;
To determine appropriate actions preceding the implementation of a change, e.g., additional testing, (re)qualification, (re)validation or communication with regulators.
Continual improvement
To facilitate continual improvement in processes throughout the product lifecycle.
II.2 Quality risk management as part of regulatory operations
II.2 药政操作部分的质量风险管理
Inspection and assessment activities
To assist with resource allocation including, for example, inspection planning and frequency, and inspection and assessment intensity (see "Auditing" section in Annex II.1);
To evaluate the significance of, for example, quality defects, potential recalls and inspectional findings;
To determine the appropriateness and type of post-inspection regulatory follow-up;
To evaluate information submitted by industry including pharmaceutical development information;
To evaluate impact of proposed variations or changes;
To identify risks which should be communicated between inspectors and assessors to facilitate better understanding of how risks can be or are controlled (e.g., parametric release, Process Analytical Technology (PAT)).
II.3 Quality risk management as part of development
II.3 开发部分的质量风险管理
To design a quality product and its manufacturing process to consistently deliver the intended performance of the product (see ICH Q8);
To enhance knowledge of product performance over a wide range of material attributes (e.g., particle size distribution, moisture content, flow properties), processing options and process parameters;
To assess the critical attributes of raw materials, solvents, Active Pharmaceutical Ingredient (API) starting materials, APIs, excipients, or packaging materials;
To establish appropriate specifications, identify critical process parameters and establish manufacturing controls (e.g., using information from pharmaceutical development studies regarding the clinical significance of quality attributes and the ability to control them during processing);
To decrease variability of quality attributes:
• reduce product and material defects;
• reduce manufacturing defects.
To assess the need for additional studies (e.g., bioequivalence, stability) relating to scale up and technology transfer;
To make use of the “design space” concept (see ICH Q8).
- 减少产品及物料的缺陷;
- 减少生产缺陷。
II.4 Quality risk management for facilities, equipment and utilities
II.4 厂房,设备和公用设施的质量风险管理
Design of facility / equipment
To determine appropriate zones when designing buildings and facilities, e.g.,
• flow of material and personnel;
• minimize contamination;
• pest control measures;
• prevention of mix-ups;
• open versus closed equipment;
• clean rooms versus isolator technologies;
• dedicated or segregated facilities / equipment.
To determine appropriate product contact materials for equipment and containers (e.g., selection of stainless steel grade, gaskets, lubricants);
To determine appropriate utilities (e.g., steam, gases, power source, compressed air, heating, ventilation and air conditioning (HVAC), water);
To determine appropriate preventive maintenance for associated equipment (e.g., inventory of necessary spare parts).
- 人流和物流;
- 污染最小化;
- 虫害控制措施;
- 防止混淆;
- 开放式设备相对于密闭式设备;
- 洁净室相对于隔离器技术;
- 专用的或区隔的厂房设施、设备。
Hygiene aspects in facilities
To protect the product from environmental hazards, including chemical, microbiological, and physical hazards (e.g., determining appropriate clothing and gowning, hygiene concerns);
To protect the environment (e.g., personnel, potential for cross-contamination) from hazards related to the product being manufactured.
Qualification of facility/equipment/utilities
To determine the scope and extent of qualification of facilities, buildings, and production equipment and/or laboratory instruments (including proper calibration methods).
Cleaning of equipment and environmental control
To differentiate efforts and decisions based on the intended use (e.g., multi- versus single-purpose, batch versus continuous production);
To determine acceptable (specified) cleaning validation limits.
Calibration/preventive maintenance
To set appropriate calibration and maintenance schedules.
Computer systems and computer controlled equipment
To select the design of computer hardware and software (e.g., modular, structured, fault tolerance);
To determine the extent of validation, e.g.,
• identification of critical performance parameters;
• selection of the requirements and design;
• code review;
• the extent of testing and test methods;
• reliability of electronic records and signatures.
- 关键性能参数的识别;
- 要求与设计的选择;
- 源代码的审核;
- 测试程度和测试方法;
- 电子记录及电子签名的可靠性。
II.5 Quality risk management as part of materials management
II.5 物料管理部分的质量风险管理
Assessment and evaluation of suppliers and contract manufacturers
To provide a comprehensive evaluation of suppliers and contract manufacturers (e.g., auditing, supplier quality agreements).
Starting material
To assess differences and possible quality risks associated with variability in starting materials (e.g., age, route of synthesis).
Use of materials
To determine whether it is appropriate to use material under quarantine (e.g., for further internal processing);
To determine appropriateness of reprocessing, reworking, use of returned goods.
Storage, logistics and distribution conditions
To assess the adequacy of arrangements to ensure maintenance of appropriate storage and transport conditions (e.g., temperature, humidity, container design);
To determine the effect on product quality of discrepancies in storage or transport conditions (e.g., cold chain management) in conjunction with other ICH guidelines;
To maintain infrastructure (e.g., capacity to ensure proper shipping conditions, interim storage, handling of hazardous materials and controlled substances, customs clearance);
To provide information for ensuring the availability of pharmaceuticals (e.g., ranking risks to the supply chain).
II.6 Quality risk management as part of production
II.6 生产部分的质量风险管理
Validation
To identify the scope and extent of verification, qualification and validation activities (e.g., analytical methods, processes, equipment and cleaning methods;
To determine the extent for follow-up activities (e.g., sampling, monitoring and re-validation);
To distinguish between critical and non-critical process steps to facilitate design of a validation study.
In-process sampling & testing
To evaluate the frequency and extent of in-process control testing (e.g., to justify reduced testing under conditions of proven control);
To evaluate and justify the use of process analytical technologies (PAT) in conjunction with parametric and real time release.
Production planning
To determine appropriate production planning (e.g., dedicated, campaign and concurrent production process sequences).
II.7 Quality risk management as part of laboratory control and stability studies
II.7 实验室控制和稳定性研究部分的质量风险管理
Out of specification results
To identify potential root causes and corrective actions during the investigation of out of specification results.
Retest period / expiration date
To evaluate adequacy of storage and testing of intermediates, excipients and starting materials.
II.8 Quality risk management as part of packaging and labelling
II.8 包装和标签部分的质量风险管理
Design of packages
To design the secondary package for the protection of primary packaged product (e.g., to ensure product authenticity, label legibility).
Selection of container closure system
To determine the critical parameters of the container closure system.
Label controls
To design label control procedures based on the potential for mix-ups involving different product labels, including different versions of the same label.
II.9 Quality Risk Management as Part of Supply Chain Control
II.9 供应链控制中的质量风险管理
With regard to product availability risks related to quality/manufacturing issues, product lifecycle oversight of the supply chain includes maintaining current knowledge of quality/manufacturing hazards and prioritizing efforts to manage such risks. Understanding hazards to quality/manufacturing is critical to maintaining supply predictability. When risks are well understood and controlled, a higher confidence in product availability can be attained.
Manufacturing Process Variation and State of Control
To decrease variability in the manufacturing process (e.g., process drift, non-uniformity) and associated capability gaps that can result in unpredictable outputs, adversely impact quality and consequently timeliness, yield and product availability;
To design monitoring systems that are capable of detecting departures from a state of control and deficiencies in manufacturing processes, so they can be appropriately investigated to determine root causes and any required risk mitigations.
Manufacturing Facilities and Equipment
To ensure that facility infrastructure and equipment are suitable and designed for robust manufacturing (this includes packaging and testing) (see Annex II.4);
To establish facility and equipment maintenance programmes that assure reliable facility and equipment performance;
To ensure that the operational design of equipment is not vulnerable to human error;
To obtain quality and efficiency gains through the utilization of digitalization, automation, isolation technology, and other innovations.
Supplier Oversight and Relationships
To enhance review and monitoring activities (see Section 2.7 of ICH Q10) when substantial 24ICH Q9(R1) Guideline variability is identified in the quality and safety of supplied materials or in the services provided.
To manage external product availability risks relating to quality/manufacturing, (e.g., from raw material suppliers, contracted organizations, service providers, etc.)